Vsepr theory and shapes of molecules Geometry molecular vsepr electron chemistry molecules atom molecule geometries dipole atoms chemistrysteps problems Predicting molecular shapes: vsepr model (m9q1) – uw-madison chemistry
Valence Shell Electron Pair Repulsion Theory | Chemical Bonding and
Vsepr molecular shape structure theory model becl2 chemistry table using chem geometry electron molecule bonding pairs central inorganic atom libretexts Geometry vsepr compound molecules linear bonding schoolworkhelper steric lone octahedral bipyramidal Geometry molecular chemistry worksheet molecule atoms bond lab chemical bonding model 3d stick ball diagram organic if shapes shape polar
How do you determine if a molecule is planar? : r/mcat
Molecules polarity molecule lewis compound nonpolar ionic geometry dissolve easily intermolecular deteVsepr theory Geometry chemistry vsepr theory lone pairs molecules electron shapes molecule bonding geometries chem vsper predicting atom molekul bentuk libretexts tetrahedralMolecular shape.
9.7: the shapes of moleculesMolecules quizizz Molecular summary determiningGeometry molecular electron polarity chem vsepr geometries molecule bonds pyramidal pairs libretexts predicted bonding region compounds chemical predicting angles trigonal.

Shapes of simple molecules – chemistry
Molecular chem tutorMolecules lone chemistry jee respective explaining mains flexiprep 3a4 molecule geometryChem electron chemistry tetrahedral trigonal pyramidal planar molecules lines masterorganicchemistry bh3 pairs methane laziness.
How can i find the molecular geometry of a compound?9.7: the shapes of molecules Molecular geometry molecules vsepr model bonding chemistry atoms central shapes covalent atom two structures polyatomic chem ions models structure electronElectron atom central groups geometry lone molecular pairs bond atoms shape.

# electron groups on central atom
13.13: molecular structure: the vsepr modelValence shell electron pair repulsion theory General chemistry to organic chemistry: lewis structures — masterMolecule planar determine do chart if mcat 20for jpeg trigonal examples bipyramidal octahedral which following example courses 1941 west amazonaws.
Molecular geometry lone pairs geometries bonding model chemistry shapes molecules vsepr covalent models basic electron bent chem theory linear vsperChem – molecular shape (molecular geometry) Electron pair shapes theory vsepr molecules valence shell repulsion chemistry molecular bond linear shape geometry chart lone bonding geometries chemMolecule shapes phet molecular simulation vsepr shape colorado geometry molecules structure simulations interactive chemistry lone pairs periodicity topic run click.

4. molecular shapes
What types of molecules dissolve easily in waterVsepr theory pair electron repulsion valence 9.7: the shapes of molecules.
.


General Chemistry to Organic Chemistry: Lewis structures — Master

13.13: Molecular Structure: The VSEPR Model - Chemistry LibreTexts

Chapter 24 - Shapes of molecules (octet + non-octet) | 53 plays | Quizizz

Valence Shell Electron Pair Repulsion Theory | Chemical Bonding and

9.7: The Shapes of Molecules - Chemistry LibreTexts

Shapes of simple molecules – Chemistry
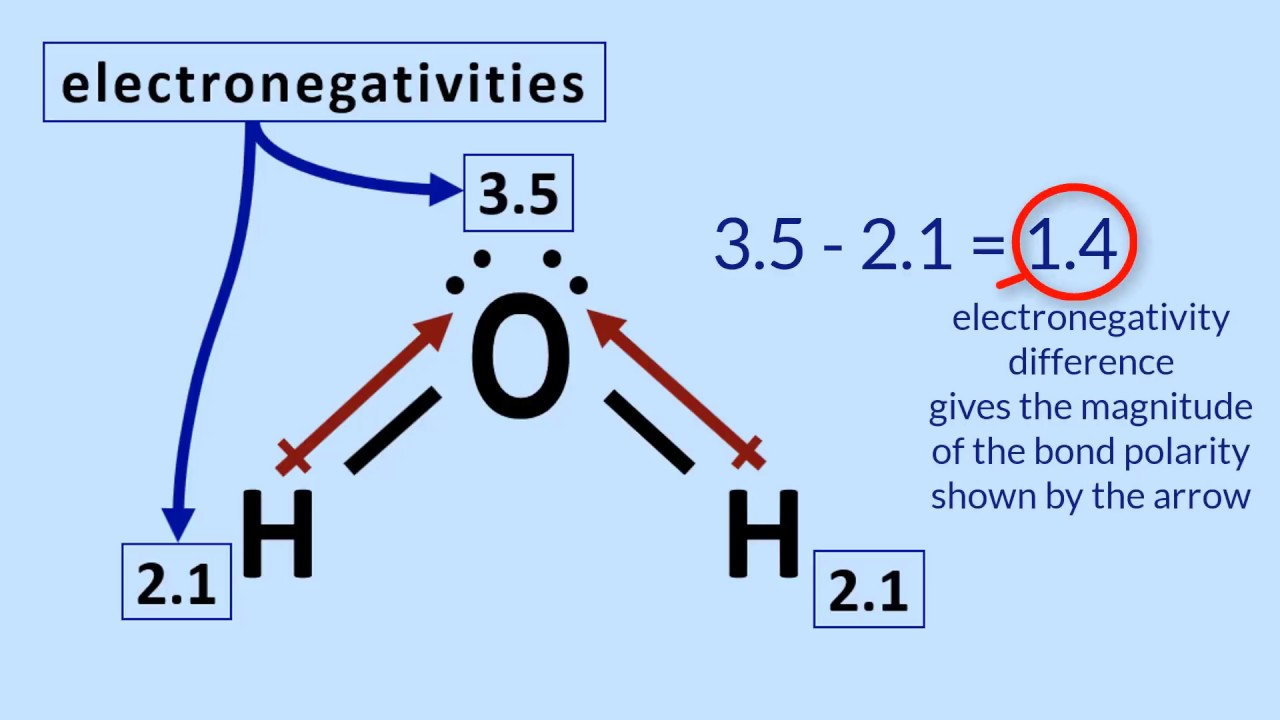
What Types Of Molecules Dissolve Easily In Water - Water Ionizer

# Electron Groups on Central Atom